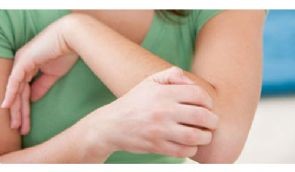
AKP-11 is being developed as a treatment for psoriasis. AKP-11 has been tested on a small population of psoriasis subjects previously, which showed a reduction in psoriasis severity. By participating in this study you will help to obtain important information about whether AKP-11 is safe, tolerable and effective in the treatment of psoriasis.
You will be required to attend the clinic for a screening visit so that the study doctor can decide whether you are eligible to participate. In this study, one out of two will receive a topical administration of 3% AKP-11 twice a day for up to 42 days and one out of two will receive topical administration of a placebo twice a day. If you are eligible, you will be included in one of these two treatment groups. After screening, you will be required to attend the clinic a total of six times plus an additional visit (optional).
You can register your interest for the trial via the CTC website at ctc.asn.au or by contacting our friendly staff.
If you are interested and would like more information, please contact:
Sara Collis – Study Coordinator
Phone: 96239400/96239406
Email scollis@skincancer.asn.au
Website: https://www.ctc.asn.au/trials/mildpsoriasis
Eligible participants in this clinical study will receive reimbursement for their time and travel. Bellberry Ethics Committee has approved this study.