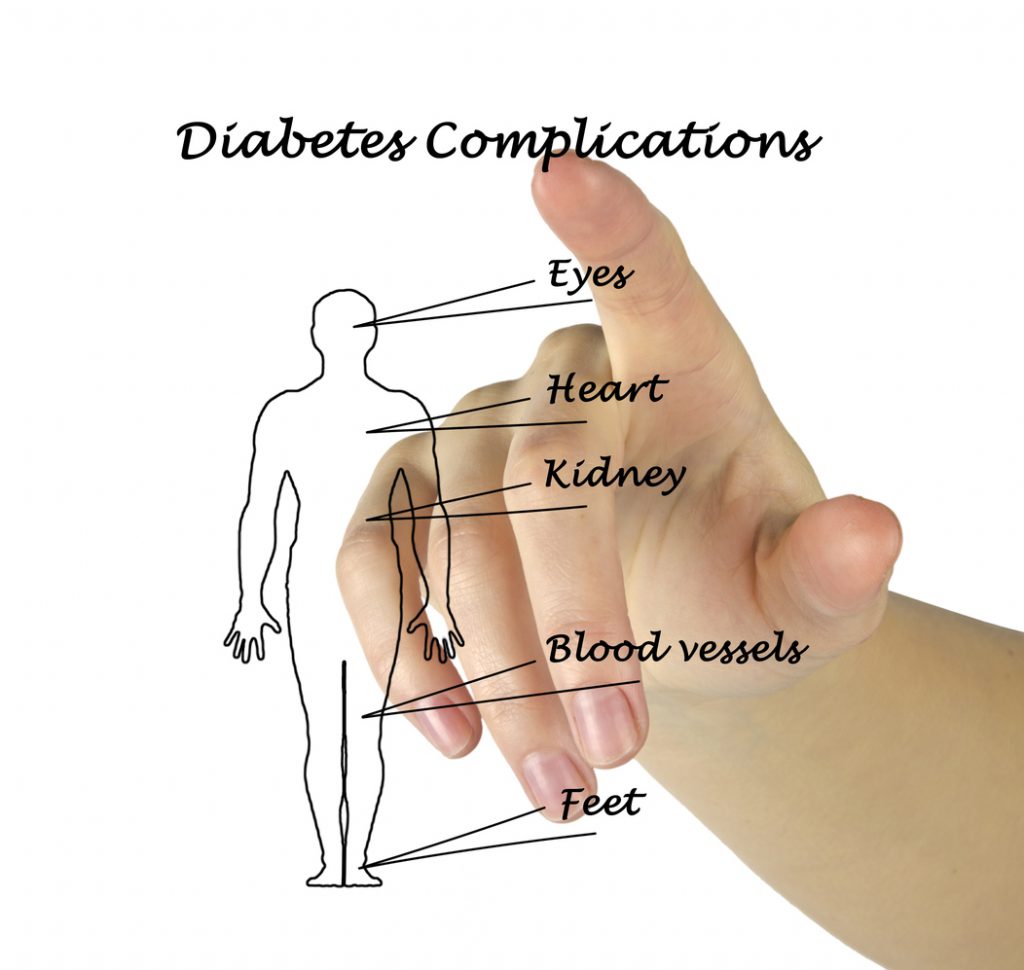
A 26 Week, Multicenter, Randomized, Placebo-Controlled, Double-Blind, Parallel Group, Phase 3 Trial With a 26 Week Safety Extension Period Evaluating the Safety and Efficacy of Dapagliflozin 5 and 10 mg, and Saxagliptin 2.5 and 5 mg in Pediatric Patients With Type 2 Diabetes Mellitus Who Are Between 10 and Below 18 Years of Age.